HCOOCH CH2 H2O: Complete Laboratory Synthesis Protocol with Troubleshooting Guide

Introduction
The synthesis of HCOOCH CH2 H2O represents one of the most fundamental yet industrially significant reactions in organic chemistry. Understanding how to properly conduct methyl formate hydrolysis in a laboratory setting is essential for chemists working in pharmaceutical synthesis, green chemistry research, and industrial process development. Despite the widespread use of HCOOCH CH2 H2O reactions in producing formic acid and methanol, comprehensive laboratory protocols with practical troubleshooting guidance remain surprisingly scarce in accessible literature.
This complete guide provides chemistry professionals, graduate students, and industrial researchers with a detailed, step-by-step protocol for synthesizing and optimizing HCOOCH CH2 H2O reactions in laboratory environments. Whether you’re conducting academic research, optimizing industrial processes, or training new laboratory personnel, this protocol delivers the practical insights needed to achieve consistent, high-yield results while maintaining rigorous safety standards.
Throughout this guide, we’ll explore the chemical principles underlying HCOOCH CH2 H2O synthesis, provide detailed experimental procedures, address common challenges through systematic troubleshooting approaches, and demonstrate quality control methods that ensure reproducible outcomes.
Understanding HCOOCH CH2 H2O Chemistry
Molecular Structure and Reaction Fundamentals
HCOOCH CH2 H2O describes the hydrolysis reaction system involving methyl formate (HCOOCH₃), methylene intermediates (CH₂), and water (H₂O). This chemical transformation represents a classic example of ester hydrolysis, where the ester bond in methyl formate undergoes nucleophilic substitution to yield formic acid (HCOOH) and methanol (CH₃OH).
The fundamental reaction proceeds as follows:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
This seemingly simple transformation involves complex mechanistic pathways that depend heavily on reaction conditions, catalyst selection, and environmental factors. Understanding the molecular-level interactions within HCOOCH CH2 H2O systems enables chemists to predict reaction behavior and optimize synthesis parameters for specific applications.
Mechanistic Pathways
The hydrolysis of methyl formate in HCOOCH CH2 H2O systems can proceed through two distinct mechanistic routes:
Acid-Catalyzed Mechanism: In acidic conditions, protonation of the carbonyl oxygen enhances the electrophilic character of the carbonyl carbon. Water molecules then perform nucleophilic attack, forming a tetrahedral intermediate. Subsequent proton transfers and bond cleavage release methanol and formic acid. This pathway proves reversible and reaches equilibrium under standard conditions.
Base-Catalyzed Mechanism: Under basic conditions, hydroxide ions directly attack the carbonyl carbon, forming an anionic tetrahedral intermediate. This mechanism proceeds irreversibly to completion, yielding formate ions and methanol. Subsequent acidification converts formate ions to formic acid.
Understanding these mechanistic differences proves crucial when designing HCOOCH CH2 H2O synthesis protocols, as catalyst choice dramatically impacts reaction kinetics, equilibrium position, and product purity.
Thermodynamic Considerations
The HCOOCH CH2 H2O hydrolysis reaction exhibits moderate exothermicity (ΔH ≈ -15 kJ/mol), releasing heat during the transformation. This exothermic character necessitates careful temperature control in laboratory syntheses, particularly when scaling reactions beyond analytical quantities. Entropy changes favor product formation due to the increase in molecular species, making the reaction thermodynamically favorable at standard temperatures.
Equipment and Materials Required
Essential Laboratory Equipment
Primary Reaction Vessel:
- Round-bottom flask (250 mL to 1 L, depending on scale)
- Three-neck flask is preferred for temperature monitoring and addition control
- Ground glass joints (24/40 standard)
Temperature Control:
- Heating mantle or oil bath with precise temperature control (±2°C)
- Thermometer or thermocouple probe (0-150°C range)
- Reflux condenser with circulating water supply
Addition and Sampling:
- Pressure-equalizing addition funnel (100-250 mL)
- Septum-sealed ports for sampling
- Gas-tight syringes for liquid transfer (1-10 mL)
Monitoring Equipment:
- pH meter with temperature compensation
- Magnetic stirrer with heating capability (600-1200 rpm)
- Teflon-coated stir bars (appropriate size for vessel)
Safety and Separation:
- Fume hood with adequate airflow (minimum 100 fpm face velocity)
- Separatory funnel for liquid-liquid extraction
- Distillation apparatus for product purification
Chemical Reagents
Primary Reactants:
- Methyl formate (HCOOCH₃): 99.5% purity, ACS grade
- Deionized or distilled water (H₂O): 18.2 MΩ·cm resistivity
- Sulfuric acid (H₂SO₄): Concentrated, 95-98% (for acid-catalyzed route)
- Sodium hydroxide (NaOH): Pellets or 10 M solution (for base-catalyzed route)
Analytical Standards:
- Formic acid standard (HCOOH): 88-90% aqueous solution
- Methanol standard (CH₃OH): HPLC grade, 99.9%
- Internal standards for chromatographic analysis
Safety and Neutralization:
- Sodium bicarbonate (NaHCO₃): Powder for spill neutralization
- Calcium chloride (CaCl₂) or sodium sulfate (Na₂SO₄): Anhydrous drying agents
Personal Protective Equipment
Working with HCOOCH CH2 H2O synthesis requires comprehensive PPE:
- Chemical-resistant gloves (nitrile, 6 mil minimum thickness)
- Safety goggles with side shields or a full-face shield
- Laboratory coat (flame-resistant, 100% cotton)
- Closed-toe shoes (chemical-resistant preferred)
- Fume hood operation for all synthesis steps
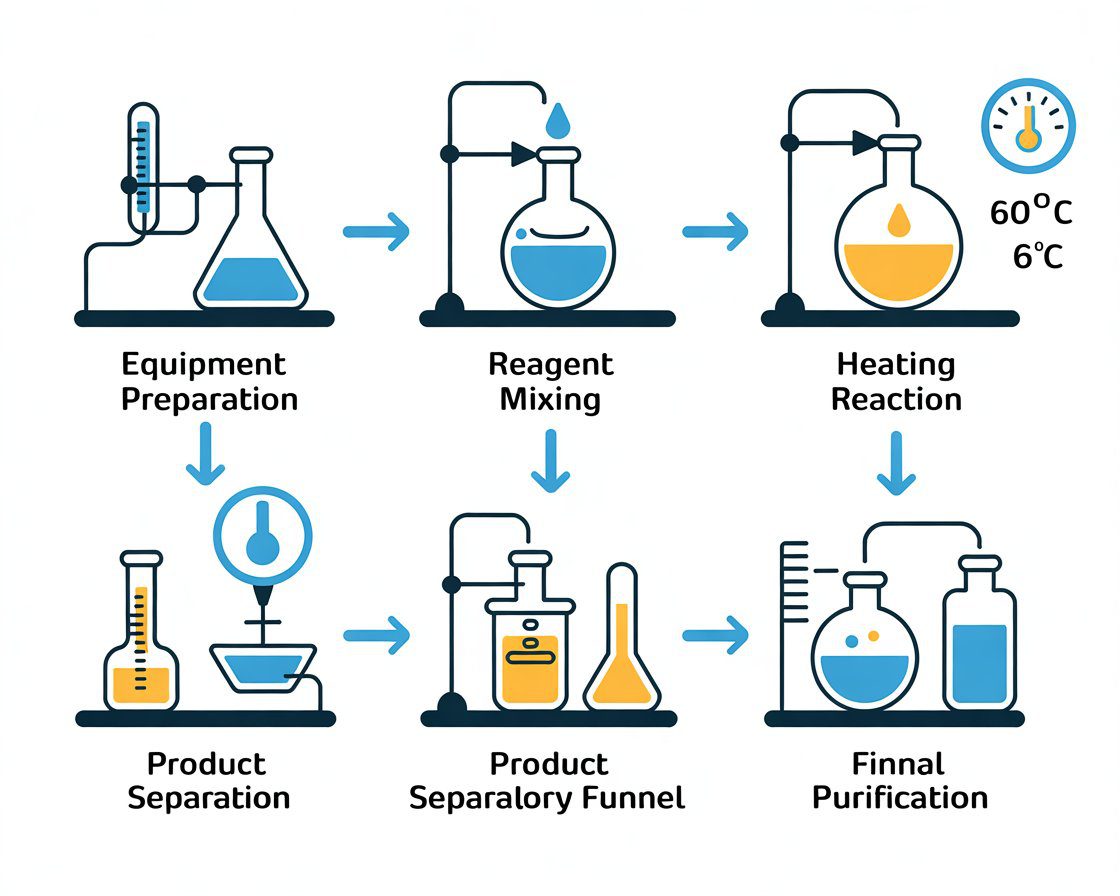
Pre-Reaction Preparation
Step 1: Safety and Workspace Setup (15 minutes)
Begin by establishing a safe working environment. Verify fume hood functionality by checking face velocity with an anemometer; readings should exceed 100 feet per minute for adequate ventilation. Position all equipment within the hood, ensuring the sash remains at the designated safe operating height throughout the procedure.
Inspect all glassware for cracks, chips, or defects that could compromise safety during HCOOCH CH2 H2O synthesis. Pay particular attention to ground glass joints, ensuring clean surfaces free from contamination or damage. Assemble the reaction apparatus before introducing any chemicals.
Step 2: Reagent Preparation and Measurement (20 minutes)
For a standard 0.5 mol scale HCOOCH CH2 H2O synthesis:
Measure 30.0 mL (28.8 g, 0.48 mol) of methyl formate using a graduated cylinder in the fume hood. Transfer to the addition funnel. Methyl formate’s volatility requires rapid transfer to minimize evaporative losses.
Prepare the aqueous acid solution by slowly adding 2.5 mL of concentrated sulfuric acid to 100 mL of deionized water in a heat-resistant container. Always add acid to water, never the reverse, to prevent dangerous exothermic reactions. Allow this solution to cool to room temperature before proceeding.
Step 3: Initial Charge and System Assembly (10 minutes)
Add the prepared acid solution to the 500 mL three-neck round-bottom flask. Insert a Teflon-coated magnetic stir bar sized appropriately for the vessel (typically 30-40 mm length). Connect the reflux condenser to the central neck, ensuring secure ground glass joints sealed with vacuum grease if necessary.
Mount the addition funnel containing methyl formate to one side neck. Insert a thermometer or thermocouple probe through the remaining neck, positioning the temperature sensor in the liquid phase without touching the flask bottom. Begin stirring at 400 rpm to establish circulation.
Reaction Execution
Step 4: Temperature Equilibration and Catalyst Activation (15 minutes)
Before introducing methyl formate, heat the aqueous acid solution to the target temperature of 60°C using controlled heating. This temperature represents an optimal balance between reaction rate and controllability for HCOOCH CH2 H2O synthesis. Monitor temperature carefully during heating—the acid-catalyzed hydrolysis mechanism becomes active only after reaching working temperature.
Maintain steady stirring throughout heating to ensure uniform temperature distribution. Temperature gradients within the reaction mixture can create localized hot spots that promote unwanted side reactions or reduce overall yield.
Step 5: Methyl Formate Addition and Initial Reaction Phase (45-60 minutes)
Once the reaction mixture stabilizes at 60°C (±2°C), begin dropwise addition of methyl formate from the addition funnel. Control the addition rate to maintain gentle reflux without excessive vapor production. Target an addition rate of approximately 0.5 mL per minute.
During this phase, the HCOOCH CH2 H2O reaction proceeds actively, generating heat from the exothermic hydrolysis. Monitor temperature continuously. If the temperature exceeds 65°C, slow or pause methyl formate addition until the temperature decreases. The goal is to maintain steady-state conditions where heat generation from reaction balances heat removal through the condenser.
Observe the reflux condenser throughout the addition. Properly controlled reactions show steady condensate droplets returning to the flask without excessive vapor escape. If you observe vapor bypassing the condenser, reduce heating and check the cooling water flow rate.
Step 6: Extended Reaction Period and Equilibrium Achievement (90-120 minutes)
After completing methyl formate addition, maintain the reaction mixture at 60°C with continuous stirring for an additional 90-120 minutes. During this period, the HCOOCH CH2 H2O equilibrium shifts toward products as the acid-catalyzed mechanism drives ester hydrolysis.
Sample the reaction mixture every 30 minutes using a gas-tight syringe. Withdraw 0.5 mL samples through the septum port, quench immediately in 5 mL of cold deionized water, and analyze by gas chromatography (GC) or high-performance liquid chromatography (HPLC) to monitor conversion progress.
Reaction completion is indicated when methyl formate concentration stabilizes below 5% of the initial loading. Typical reaction times range from 120-180 minutes under these conditions, though exact timing varies based on catalyst concentration and temperature control precision.
Product Isolation and Purification
Step 7: Reaction Quenching and Neutralization (20 minutes)
Upon reaching target conversion, cool the reaction mixture to room temperature using an ice bath while maintaining stirring. This cooling step is essential for safe handling and prevents decomposition during subsequent neutralization.
For acid-catalyzed HCOOCH CH2 H2O syntheses, carefully neutralize excess acid by slowly adding saturated sodium bicarbonate solution with vigorous stirring. Add bicarbonate solution dropwise initially, as carbon dioxide evolution can cause foaming. Monitor pH continuously, target a final pH of 6.5-7.0 for optimal product stability.
Step 8: Liquid-Liquid Extraction and Drying (45 minutes)
Transfer the neutralized reaction mixture to a separatory funnel. Add 100 mL of diethyl ether or ethyl acetate to extract organic products. Shake vigorously for 2 minutes, venting pressure regularly through the stopcock. Allow phases to separate (5-10 minutes). The upper organic layer contains methanol and residual methyl formate, while the lower aqueous layer holds formic acid.
Drain the aqueous phase containing formic acid into a clean flask. Wash the organic layer twice with 50 mL portions of deionized water to remove residual formic acid contamination. Combine all aqueous fractions; this represents your formic acid product stream.
Dry the organic layer by adding anhydrous sodium sulfate (approximately 20 g). Swirl gently and allow to stand for 15 minutes. The drying agent removes dissolved water, preventing azeotrope formation during subsequent distillation.
Step 9: Distillation and Final Purification (60-90 minutes)
Filter the dried organic layer through filter paper to remove the drying agent. Transfer the filtrate to a clean distillation apparatus. Distill methanol at atmospheric pressure, collecting the fraction boiling between 64-66°C. This represents purified methanol recovered from HCOOCH CH2 H2O synthesis.
For formic acid purification, concentrate the combined aqueous fractions using rotary evaporation at reduced pressure (20-40 mmHg) and moderate temperature (40-50°C). Formic acid’s high boiling point (100.8°C) allows selective removal of water, yielding a concentrated formic acid solution (70-85% typical concentration).
Optimizing HCOOCH CH2 H2O Production
Temperature Optimization
Temperature exerts profound influence on HCOOCH CH2 H2O reaction kinetics and equilibrium position. While higher temperatures accelerate reaction rates following Arrhenius behavior, they simultaneously promote undesirable side reactions and reduce equilibrium conversion due to unfavorable thermodynamics.
Systematic optimization studies reveal that 60-70°C represents the optimal temperature range for acid-catalyzed methyl formate hydrolysis. Within this range, reaction rates remain sufficiently fast (90% conversion in 2-3 hours) while minimizing formic acid decomposition to carbon monoxide and water.
Catalyst Selection and Concentration
Sulfuric acid concentration significantly impacts HCOOCH CH2 H2O synthesis performance. Concentrations between 0.1-0.5 M provide optimal catalytic activity without excessive corrosion concerns or difficult neutralization requirements. Higher acid concentrations (>1.0 M) accelerate hydrolysis but complicate downstream processing and increase safety hazards.
Alternative acid catalysts include hydrochloric acid, phosphoric acid, and solid acid catalysts like Amberlyst-15 resin. Each catalyst type offers distinct advantages. Solid acids simplify product separation but may show reduced activity, while mineral acids provide maximum catalytic efficiency with more complex workup requirements.
Water Stoichiometry and Excess
Le Chatelier’s principle dictates that excess water shifts the HCOOCH CH2 H2O equilibrium toward products. Practical considerations balance this thermodynamic benefit against increased reactor volumes and energy requirements for product concentration.
Empirical studies demonstrate that water-to-methyl formate molar ratios between 3:1 and 10:1 provide optimal results. Ratios below 3:1 limit conversion due to equilibrium constraints, while ratios exceeding 10:1 offer marginal conversion improvements with substantial processing disadvantages.
Stirring and Mass Transfer
Efficient mixing proves essential for HCOOCH CH2 H2O synthesis because methyl formate and water show limited mutual solubility. Inadequate agitation creates biphasic systems with poor interfacial mass transfer, dramatically reducing reaction rates.
Maintain stirring speeds above 400 rpm for laboratory-scale reactions. In larger vessels, calculate required agitation based on the vessel geometry and fluid properties to ensure adequate mixing power numbers and Reynolds numbers provide quantitative design criteria.
Troubleshooting HCOOCH CH2 H2O Synthesis
Problem: Low Conversion (<70%)
Symptoms: After standard reaction time (2-3 hours at 60°C), GC analysis shows methyl formate concentration remains above 30% of initial loading. Formic acid and methanol yields fall below expected values.
Diagnostic Steps:
- Verify actual reaction temperature using a calibrated thermometer—thermometer positioning errors commonly cause apparent low conversions
- Check pH of reaction mixture. Insufficient acid catalyst limits the hydrolysis rate
- Analyze water content, evaporative losses reduce the effective water concentration
- Inspect the stirring efficiency phase separation indicates poor mixing
Solutions:
- Temperature Correction: If the actual temperature measures below 60°C, increase heating gradually until reaching the target temperature. Verify heating mantle calibration.
- Catalyst Addition: If pH exceeds 2.0, carefully add additional sulfuric acid (0.5 mL increments) until pH decreases to 1.0-1.5. Allow 30 minutes for equilibration after each addition.
- Water Replenishment: Add 20-30 mL deionized water to compensate for evaporative losses. Ensure condenser cooling water flows properly.
- Enhanced Mixing: Increase stirring speed to 600-800 rpm. Verify stir bar remains coupled to the magnetic stirrer occasionally; stir bars decouple at lower speeds.
- Extended Reaction Time: Continue heating for an additional 60-90 minutes, sampling every 30 minutes to monitor progress.
Problem: Product Contamination
Symptoms: GC or NMR analysis reveals unexpected peaks indicating side products. Formic acid or methanol purity falls below target specifications (>95%).
Diagnostic Steps:
- Identify contaminant species through mass spectrometry or detailed NMR analysis
- Review the reaction temperature profile; temperature excursions promote decomposition
- Check reagent quality. Impure methyl formate introduces contaminants
- Evaluate the neutralization procedure; incomplete neutralization affects product stability
Solutions:
- Temperature Excursion Prevention: Install a temperature controller with alarm functionality. Set the upper limit at 65°C to prevent runaway exothermic reactions.
- Reagent Purification: Distill methyl formate before use if contamination originates from the starting material. Collect narrow boiling fraction (31-33°C).
- Improved Workup: Perform additional washing steps during extraction. Three 30 mL washes typically remove residual impurities more effectively than a single large-volume wash.
- Purification Enhancement: If contamination persists, perform distillation under reduced pressure to separate products from high-boiling impurities.
Problem: Excessive Reaction Rate/Thermal Runaway
Symptoms: Rapid temperature increase during methyl formate addition. Vigorous boiling overwhelms the reflux condenser capacity. Vapor escapes from the condenser top.
Diagnostic Steps:
- Immediate action is required to cease methyl formate addition and remove the heating
- Apply an ice bath to cool the reaction vessel
- Check the condenser cooling water for inadequate flow causes insufficient heat removal
- Verify catalyst concentration, excess acid accelerates the reaction dangerously
Solutions:
- Emergency Cooling: Apply an ice bath while maintaining stirring. The temperature decreases to 40°C before resuming.
- Reduced Addition Rate: Decrease methyl formate addition to 0.25 mL/min or slower. Use a peristaltic pump for precise flow control.
- Lower Initial Temperature: Begin methyl formate addition at 50°C rather than 60°C, gradually increasing temperature during addition.
- Diluted Catalyst: Prepare a less concentrated acid solution (0.1 M H₂SO₄ rather than 0.25 M). Slower reaction rate improves controllability.
- Condenser Upgrade: Install a larger condenser or cooling system with greater heat removal capacity for subsequent syntheses.
Problem: Phase Separation During Reaction
Symptoms: A Clear interface visible between two liquid phases in the reaction flask. Stirring fails to create a homogeneous mixture. Reduced reaction efficiency is evident from conversion monitoring.
Diagnostic Steps:
- Observe stirring pattern, effective agitation creates a transient emulsion
- Check methyl formate addition rate; too-rapid addition overwhelms mixing capacity
- Evaluate temperature: lower temperatures reduce mutual solubility
- Inspect stir bar size. Undersized stir bars provide insufficient mixing power
Solutions:
- Enhanced Mechanical Agitation: Increase stirring speed to maximum safe level (typically 1000-1200 rpm). Consider an overhead mechanical stirrer for larger scales.
- Surfactant Addition: In extreme cases, add a small amount (<0.5% v/v) of a suitable surfactant like polyethylene glycol to improve phase compatibility. Note: surfactant addition requires additional purification steps.
- Modified Addition Strategy: Pre-dilute methyl formate with a small amount of methanol (9:1 ratio) to improve miscibility. This modification slightly alters the stoichiometry but greatly improves mixing.
- Temperature Increase: Raise reaction temperature to 65-70°C, where component miscibility improves. Monitor more carefully for side reactions at elevated temperatures.
Problem: Poor Product Recovery
Symptoms: Expected product quantities not isolated despite analytical evidence of high conversion. Material balance indicates significant losses during workup.
Diagnostic Steps:
- Account for all streams reaction mixture, aqueous washes, and organic layer
- Check for volatility losses methanol readily evaporates during concentration
- Evaluate the extraction efficiency of incomplete phase separation traps products
- Review the neutralization procedure. Excessive neutralization consumes formic acid
Solutions:
- Careful Evaporation: Perform all concentration steps under reduced pressure at controlled temperature (<40°C for methanol recovery). Use cold traps to capture volatile components.
- Extraction Optimization: Perform three separate extractions with smaller solvent volumes rather than a single large extraction. Multiple extractions recover an additional 10-15% product.
- Precise Neutralization: Add neutralizing agent dropwise while monitoring pH continuously. Target pH 6.5-7.0 rather than excess basicity over-neutralization forms additional sodium formate, reducing formic acid yield.
- Salt Addition: Add sodium chloride (10-20 g) to aqueous phases before extraction to improve phase separation through the salting-out effect.
Quality Control and Analysis
Analytical Methods for HCOOCH CH2 H2O Products
Gas Chromatography (GC) Analysis: GC provides the most practical method for monitoring HCOOCH CH2 H2O synthesis progress and quantifying product purity. Use a polar column (Carbowax or similar, 30 m length, 0.25 mm ID) with FID detection. Typical conditions: injector 200°C, detector 250°C, oven program 40°C (2 min) to 120°C at 10°C/min.
Prepare calibration curves using authentic standards of methyl formate, formic acid, and methanol across relevant concentration ranges (0.1-10% w/w). Include n-butanol as an internal standard for quantitative accuracy.
Nuclear Magnetic Resonance (NMR) Spectroscopy: Proton NMR confirms product identity and purity. Formic acid shows a characteristic singlet at δ 8.25 ppm, while methanol appears as a quartet (δ 3.35 ppm) and a doublet (δ 3.43 ppm) when dissolved in deuterated water. Integration ratios provide purity estimation when calibrated against known standards.
Titration Methods: For formic acid quantification, perform acid-base titration using a standardized 0.1 M sodium hydroxide solution with phenolphthalein indicator. This classical method provides an accurate determination of total acid content. For products containing 70-85% formic acid, expect endpoint volumes consistent with calculated concentrations based on starting material quantities.
Quality Specifications
Acceptable HCOOCH CH2 H2O synthesis yields should meet these specifications:
Formic Acid Product:
- Purity: ≥95% by GC analysis
- Appearance: Clear, colorless liquid
- Density: 1.22 ± 0.02 g/cm³ at 20°C
- Assay: 70-85% formic acid by titration
- Residual methyl formate: <2%
Methanol Product:
- Purity: ≥98% by GC analysis
- Water content: <0.5% by Karl Fischer titration
- Appearance: Clear, colorless liquid
- Boiling point: 64-66°C at 760 mmHg
- Residual formic acid: <0.5%
Documentation and Record Keeping
Maintain comprehensive laboratory notebooks documenting all HCOOCH CH2 H2O synthesis experiments. Essential records include:
- Complete reagent information (supplier, lot number, purity, expiration date)
- Precise quantities of all materials used
- Detailed procedural notes, including timing, temperatures, and observations
- Analytical results with chromatograms or spectra are retained
- Calculation of yields, conversions, and material balances
- Any deviations from standard protocol with rationale
- Safety incidents or near-misses with corrective actions implemented
This documentation proves invaluable for reproducibility, troubleshooting future reactions, and regulatory compliance in industrial settings.
Safety Considerations for HCOOCH CH2 H2O Synthesis
Chemical Hazards
Methyl Formate: This volatile ester presents multiple hazards. Its low boiling point (32°C) means it readily forms flammable vapor-air mixtures above 5°F flash point. Inhalation causes respiratory irritation, dizziness, and, at high concentrations, central nervous system depression. Skin contact produces irritation and defatting. Always work in a fume hood and minimize exposure duration.
Formic Acid: Concentrated formic acid (>70%) causes severe skin burns and eye damage. Vapors irritate the respiratory tract and can lead to pulmonary edema at sufficient concentrations. LD50 values (rat, oral) of 1.8 g/kg indicate moderate acute toxicity. Handle with appropriate PPE and have emergency eyewash and shower readily accessible.
Methanol: Beyond flammability concerns, methanol presents serious toxicity risks. As little as 10 mL can cause blindness through metabolic conversion to formaldehyde and formic acid. Ingestion of 30-100 mL may prove fatal. Use only in well-ventilated areas and never pipette by mouth.
Sulfuric Acid: Concentrated sulfuric acid reacts violently with water, generating extreme heat. Always add acid to water, never the reverse. Contact causes severe burns. Sulfuric acid chars organic materials, including skin tissue. Wear a face shield during handling and maintain a clear path to the emergency shower.
Engineering Controls
Conduct all HCOOCH CH2 H2O synthesis operations within a properly functioning chemical fume hood. Verify adequate face velocity (100-120 fpm) before beginning work. Position all equipment at least 6 inches behind the sash opening to ensure contaminant capture.
Install secondary containment around reaction vessels to capture spills. Use heating mantles designed for flammable materials never use open flames or hot plates lacking spark-proof construction.
Emergency Procedures
Spill Response: For methyl formate spills, evacuate the area and ventilate thoroughly. Allow volatile material to evaporate in a well-ventilated space (under a fume hood for small spills). Do not attempt to wash down drains. Methyl formate reacts with water and poses environmental hazards.
For acid spills, neutralize with solid sodium bicarbonate or calcium carbonate. Approach cautiously, wearing full protective equipment. Add neutralizing agent gradually to avoid excessive heat generation or foaming. Clean neutralized residue with absorbent material and dispose as hazardous waste.
Personal Exposure: If methyl formate, formic acid, or methanol contacts skin, immediately flush with copious water for 15 minutes minimum. Remove contaminated clothing. For eye contact, flush with water or saline for 15 minutes while holding eyelids open. Seek immediate medical attention for acid or methanol exposure.
If inhaling significant vapors causes dizziness, difficulty breathing, or altered consciousness, move to fresh air immediately and seek medical evaluation. Methanol toxicity requires specific medical intervention. Inform healthcare providers of potential methanol exposure.
Scaling Considerations
While this protocol targets laboratory-scale HCOOCH CH2 H2O synthesis (0.5 mol), scaling to larger volumes requires several modifications:
Heat Transfer: Larger reaction volumes exhibit reduced surface-area-to-volume ratios, decreasing heat removal efficiency. Scale-up reactions require jacketed reactors with controlled cooling to manage exothermic heat generation. Calculate the required cooling capacity based on reaction enthalpy and desired temperature control precision.
Mixing Requirements: Mechanical overhead stirrers replace magnetic stirring at scales above 2-3 liters. Calculate agitator power requirements using standard correlations. Ensure Reynolds numbers exceed 10,000 for fully turbulent mixing, critical for maintaining phase homogeneity in HCOOCH CH2 H2O systems.
Safety Considerations: Larger scales magnify hazards. Implement additional safety measures, including rupture disks, pressure relief systems, and automated temperature control with emergency shutdown capability. Conduct hazard analysis (HAZOP) before scaling beyond 10 L.
Conclusion
This comprehensive protocol provides chemistry professionals with the detailed guidance needed to successfully conduct HCOOCH CH2 H2O synthesis in laboratory settings. By following systematic procedures, maintaining careful process control, and applying structured troubleshooting approaches when challenges arise, researchers can achieve consistent, high-yield results in methyl formate hydrolysis reactions.
The HCOOCH CH2 H2O system serves as both an industrially important synthetic route for producing formic acid and methanol, and an excellent teaching example illustrating fundamental concepts in ester chemistry, acid catalysis, and reaction engineering. Understanding how to properly execute these syntheses builds foundational skills applicable across organic chemistry and chemical process development.
For researchers seeking a deeper understanding of HCOOCH CH2 H2O chemistry, including comprehensive coverage of reaction mechanisms, industrial applications, environmental considerations, and advanced synthetic strategies, this complete guide to HCOOCH CH2 H2O chemistry provides extensive technical information across all aspects of formic acid ester systems.
Success in HCOOCH CH2 H2O synthesis ultimately depends on attention to detail, careful experimental technique, and a systematic approach to optimization and troubleshooting. The protocols and guidance provided here equip both novice and experienced chemists with practical knowledge for producing high-quality products while maintaining rigorous safety standards and achieving reproducible results across multiple experimental runs.
Frequently Asked Questions
Q: What is the typical yield for HCOOCH CH2 H2O synthesis using this protocol? Following this protocol carefully, expect 85-92% combined yields of formic acid and methanol products. Yields below 80% typically indicate procedural issues requiring troubleshooting using the guidance provided above.
Q: Can I perform HCOOCH CH2 H2O synthesis without an acid catalyst? Uncatalyzed hydrolysis proceeds extremely slowly at practical temperatures, requiring days to weeks for significant conversion. Acid or base catalysis proves essential for reasonable reaction rates. Base-catalyzed routes offer alternatives to sulfuric acid discussed here.
Q: How do I safely dispose of HCOOCH CH2 H2O synthesis waste? Neutralize all acidic wastes to pH 6-8 before disposal. Collect organic solvent wastes separately in compatible containers. Follow institutional hazardous waste disposal procedures. Never pour methanol, formic acid, or organic solvents down drains.
Q: What modifications enable green chemistry improvements to HCOOCH CH2 H2O synthesis? Consider enzymatic catalysis using lipases or esterases for more environmentally benign hydrolysis. Solid acid catalysts (acidic resins) simplify product separation and reduce waste generation. Membrane separation techniques can replace energy-intensive distillation for product purification.
Q: How does temperature affect the HCOOCH CH2 H2O reaction rate? Following Arrhenius’ relationship, the reaction rate approximately doubles for each 10°C temperature increase within the 50-70°C range. However, higher temperatures also accelerate undesired side reactions and shift the equilibrium unfavorably. The recommended 60°C represents an optimal balance between rate and selectivity.

Source: HCOOCH CH2 H2O: Complete Laboratory Synthesis Protocol with Troubleshooting Guide